Stručne o vodíkových technológiách, čo sú palivové články a ich využitie

Využitie vodíka na pohon automobilu
Autá s palivovými článkami (Fuell Cell Vehicle - FCV) majú potenciál nahradiť dopravu závislú na fosílnych palivách, ktoré prispievajú ku klimatickým zmenám. FCV sú elektromobily ktoré netreba zdĺhavo nabíjať, ale môžete ich natankovať. Odstraňujú nedostatky elektromobilov spojené s hmotnosťou a životnosťou batérií, s dojazdom a dĺžkou nabíjania. Vyzerajú ako konvenčné automobily zvonku, ale zvnútra obsahujú technologicky vyspelé komponenty, ktoré v obyčajných autách nenájdete. Najvýraznejší rozdiel je v palivových článkoch, ktoré premieňajú vodík z nádrže a s kyslíkom zo vzduchu na elektrinu poháňajúcu elektromotor.
Autám na vodíkový pohon vychádza z výfuku čistá voda.
Jediným dôvodom prečo sa masovo nepoužívajú autá na vodík je nedostatok čerpacích staníc. Na tom sa však usilovne pracuje.


Využitie vodíka ako úložisko energie pre budovy a domácnosti
Využitie fotovoltiky v spojení s elektrolyzérom a palivovým článkom umožní úplnú decentralizáciu energetiky. Prostredníctvom fotovoltických panelov si domácnosť môže vyrábať vlastnú elektrinu a prebytok, ktorý nespotrebuje použije na výrobu vodíka elektrolýzou a uskladní. Z vodíka potom pomocou palivových článkov vyrába elektrinu a používa v čase kedy panely nevyrábajú elektrinu. Batérie sa vybíjajú, ale vodík môže byť uskladnený ľubovoľný čas. Uskladnený vodík vykompenzuje nedostatok slnečného žiarenia v zimnom období.
Vyrobený vodík je tiež možné priamo tankovať do automobilu s palivovými článkami.
Na obrázku rezidencia piatich domov, ktoré si vyrábajú energiu zo slnka a uskladňujú ju vo forme vodíka.

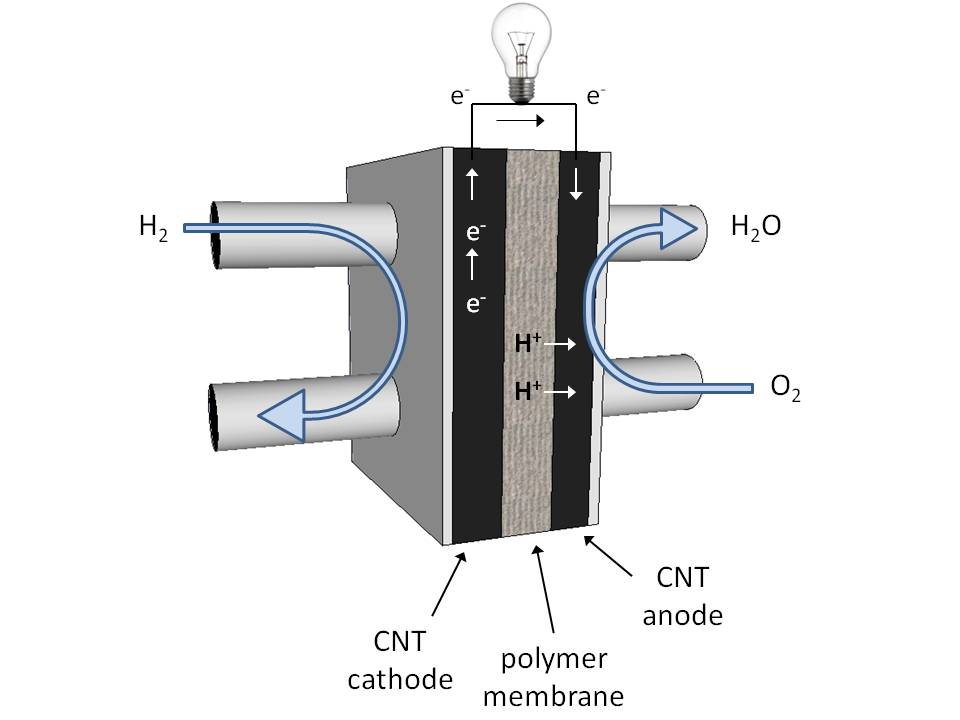
Princíp palivového článku
Viete čo je elektrolyzér? Ak áno, tak palivový článok pracuje presne opačne. Spája vodík a kyslík na vodu a vytvára pritom elektrickú energiu. Aj keď sa to teda volá palivový článok, v skutočnosti tu neprebieha spaľovanie plameňom pri vysokej teplote, ale iba katalytická reakcia pri nízkej teplote.
Palivom tu je vodík a kyslík. Ten je v bežných podmienkach v podobe molekúl, ktoré sa privádzajú na špeciálnu membránu. Na jej povrchu sa molekuly plynov vplyvom katalyzátora štiepia na atómy a ióny. Protóny vodíka, ktoré elektróde odovzdali svoj elektrón, prechádzajú cez membránu k opačnej elektróde, kde sa zlučujú s aniónmi kyslíka za vzniku molekuly vody. Elektróny pritom prechádzajú cez vonkajší elektrický obvod a sú zdrojom elektrickej energie.
Aj keď je pravda, že palivové články a vodíkové technológie sú pre mnohých neznáme, mnohé spoločnosti už majú vyvinuté komerčné produkty založené na tejto technológii. Je len otázkou času kedy sa dostupnosť a cena palivových článkov dostane na takú úroveň, že vodíkové technológie budeme využívať každý deň.
Pre lepšie pochopenie kliknite na obrázok.